| Specification of Dental Bib | |||||||||||
| Model NO. | VDB01 | Logo | OEM | ||||||||
| Material | 1ply or 2ply tissue and 1ply PE | Feature | Waterproof, soft, light, non-toxic, breathable, comfortable and easy to wear | ||||||||
| Trademark | VMED | Color | White, Green, Blue, Yellow, Purple, Pink | ||||||||
| Application | Dental Clinic | Size | 33*45cm | ||||||||
| Specification | CE, ISO, FDA | Storage | Stored in dry, humidity below 80%, ventilated, non-corrosive gases warehouse | ||||||||
| Loading Port | Wuhan | Quality Guarantee Period | 5 Years | ||||||||
| Transport Package | 125PCS/Bag, 500PCS/CTN | Weight | Paper: 16-19gsm, Film: 12-14gsm | ||||||||
| HS Code | 4818900000 | Origin | China | ||||||||
Where needs Apron

Details

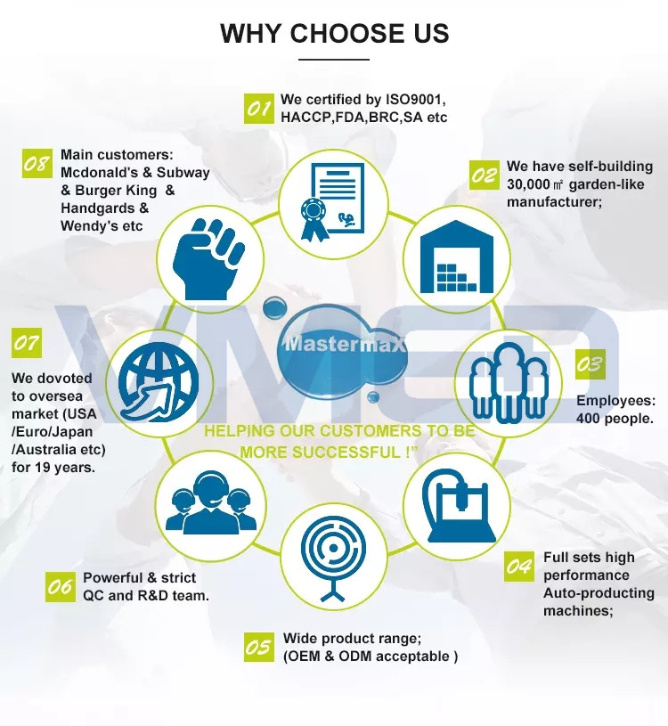
Why choose us

Company Information

Product Display






we guarantee
Goals: To take High quality ,quote most competitive price ,confirm on time delivery ,provide excellent service for customers
Characteristics:
1.Ambidextrous-fit either hand
2.Available in low and high density polyethylene
3.Various thickness, textured or smooth surface
4.Available standard length and extra-length
5.Excellent for house keeping,Food cleaning,or other .
6.Helps protect hands and fingers from contact with bacterial and other contaminants.
Assurance :
(1)Using advanced machine, fast production ,Satisfactory service.
(2) Colourful and different sizes you can choose
(3) Personal logos printing are available
(4) Professional quality assurance
FAQ
Q1: Can I have a sample first ?
VMED:Of course ! Sample are available and free
Q2: Do your company have any quality management system such as ISO, EN or CE certificate(s)?
VMED: Yes, we have CE, ISO13485, FDA,
Q3:What's your advantage? Why we choose you?
VMED: Vench Medical Products Co., Ltd. is a fast growing company, specializing in the manufacture of disposable medical products and personal protection products.
Our company has implemented a strict quality management system (QMS) and has passed ISO13485 certification. Our main products have passed CE certification of European Union (EU) and FDA registration of USA.
Q4 : How does your factory control the quality?
VMED : All manufacturing are absolutely base on the pre-production samples. Meanwhile, we have a complete after-sales system.
Q5: What the expiration date for disposable non-woven products?
VMED: We suggest 2 years of shelf storage
Trust us
-- professional &safety
Tel:+86 514 87770905
Fax:+86 514 87770905